Subtotal $0.00

Shopping cart
AaRiss DEB
- Home
- AaRiss DEB
Catheter
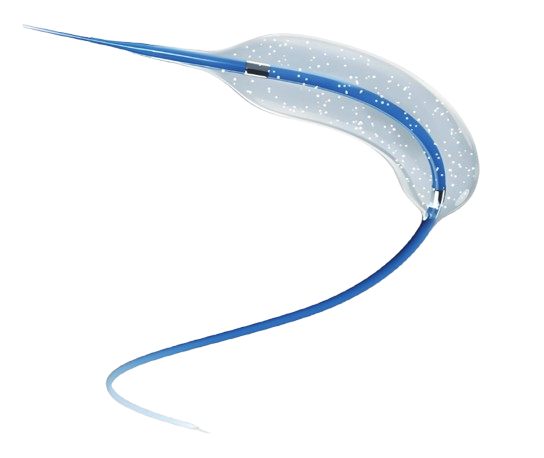
- Clinical evidence shows a low rate of restenosis
- The unique paclitaxel coating technology reduces drug loss and achieves a high release rate in target vessels
- Non-expedition design
- Different drug dose can be used for coronary artery stenosis (3µg/mm²) and intracranial artery stenosis (2µg/mm²)
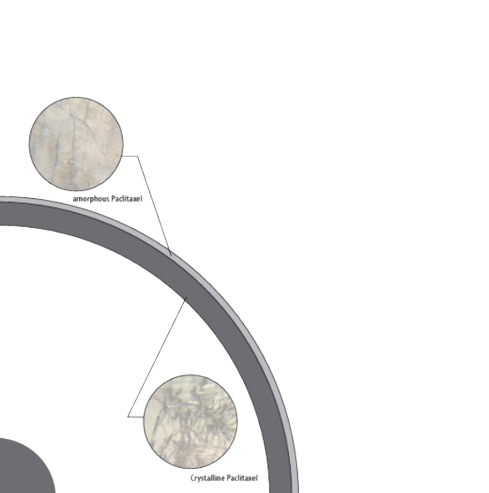
optimized coating formulation
The PurePac™ coating technology provides an optimal delivery mechanism, ensuring minimal drug loss during tracking and inflation, rapid drug transfer to the vessel wall and a sustained therapeutic effect.
- Drug: paclitaxel anti-proliferative agent
- Dose: coronary artery stenosis (3µg/mm²) and intracranial artery stenosis (2µg/mm²)
- Coating: pure paclitaxel, non-excipient design
- Layers: composite coating, 70% crystalline and 30% amorphous

Safe and effective
AaRiss™ drug-coated balloon catheter has undergone clinical trials for in-stent restenosis of coronary arteries, coronary bifurcation lesions, and intracranial atherosclerotic stenotic lesions, which have proven to inhibit restenosis while maintaining a high safety profile.
In-stent restenosis of coronary arteries (RCT)
9-month in-segment late lumen loss
AaRiss™ significantly reduced 9-month in-segment late lumen loss compared to the control group. 50% reduction.
In-stent restenosis of coronary arteries (RCT)
12-month MACE events
AaRiss™ significantly reduced 12-month MACE events compared to the control group. 67% reduction.
Coronary bifurcation lesions (RCT)
9-month diameter stenosis degree
AaRiss™ significantly reduced 9-month diameter stenosis degree compared to the control group. 24% reduction.
Coronary bifurcation lesions (RCT)
12-month MACE events
The 12-month MACE events of AaRiss™ are at the same level as the control group. 3% reduction.
Intracranial atherosclerotic stenotic lesions (Single-arm)
6-month target lesion restenosis rate
AaRiss™ 6-month target lesion restenosis rate is 8.11%, lower than that of the known drug-coated balloons used in single-arm clinical trials.
Intracranial atherosclerotic stenotic lesions (Single-arm)
6-month stroke incidence rate
AaRiss™ 6-month stroke incidence rate is 4.05%, lower than that of the known drug-coated balloons used in single-arm clinical trials.
Compliance Data
| Balloon Diameter (mm) | Pressure | |||||
|---|---|---|---|---|---|---|
| 608 kPa (6 atm) | 811 kPa (8 atm) | 1013 kPa (10 atm) | 1216 kPa (12 atm) | 1419 kPa (14 atm) | 1621 kPa (16 atm) | |
| 2.00 | 2.00 | 2.04 | 2.08 | 2.12 | 2.16 | 2.20 |
| 2.25 | 2.25 | 2.30 | 2.35 | 2.40 | 2.44 | 2.48 |
| 2.50 | 2.50 | 2.55 | 2.60 | 2.65 | 2.70 | 2.75 |
| 2.75 | 2.75 | 2.81 | 2.87 | 2.93 | 2.99 | 3.05 |
| 3.00 | 3.00 | 3.07 | 3.14 | 3.21 | 3.28 | 3.35 |
| 3.25 | 3.25 | 3.32 | 3.39 | 3.46 | 3.53 | 3.60 |
| 3.50 | 3.50 | 3.58 | 3.66 | 3.74 | 3.80 | 3.87 |
| 3.75 | 3.75 | 3.83 | 3.91 | 3.99 | 4.06 | 4.13 |
| 4.00 | 4.00 | 4.08 | 4.16 | 4.24 | 4.32 | 4.40 |
Ordering Information
| Balloon Diameter (mm) | Balloon Length (mm) |
|---|---|
| 2.0 | 06, 08, 10, 12, 15, 20, 35, 40 |
| 2.25 | 06, 08, 10, 12, 15, 20, 25, 30, 35, 40 |
| 2.5 | 06, 08, 10, 12, 15, 20, 25, 30, 35, 40 |
| 2.75 | 06, 08, 10, 12, 15, 20, 25, 30, 35, 40 |
| 3.0 | 06, 08, 10, 12, 15, 20, 25, 30, 35, 40 |
| 3.25 | 06, 08, 10, 12, 15, 20, 25, 30, 35, 40 |
| 3.5 | 06, 08, 10, 12, 15, 20, 25, 30, 35, 40 |
| 3.75 | 06, 08, 10, 12, 15, 20, 25, 30, 35, 40 |
| 4.0 | 06, 08, 10, 12, 15, 20, 25, 30, 35, 40 |
