Subtotal $0.00

Shopping cart
Home

The Celosia Coronary Covered Stent is indicated for use in the treatment of free perforations, defined as free contrast extravasation into the pericardium, in native coronary vessels ≥2.25mm in diameter.
Explore Cutting-Edge
Celosia™ Covered Stent
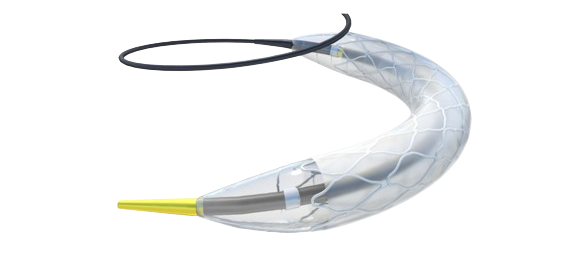

Discover a comprehensive range of covered stents designed to elevate patient care, including the latest innovations like Celosia™ and Silene™. Explore now for groundbreaking solutions in vascular intervention.
Explore Cutting-Edge
Silene™ Covered Stent


The Siro Drug Eluting Stent combines the U.S. patented Direct-Stent® design, the proven success of Sirolimus drug to reduce tissue ingrowth and proliferation, leading to reduced restenosis.

Explore Cutting-Edge
Siro™ Covered Stent

The KAURE™ Covered Stent is designed to enhance airway patency within the tracheobronchial tree. It is indicated for the treatment of tracheobronchial strictures caused by malignant neoplasms. The device is intended for use by qualified physicians experienced in diagnostic and interventional procedures, utilizing standard techniques for tracheobronchial stent placement.
Coming Soon!
Kauree™ Covered Stent

OUR DEVICES
Celosia™
Covered Stent
InformationSilene™
Covered Stent
InformationKauree™
Covered Stent
InformationSiro™
DES Stent
InformationCoronaric Stent-Graft Deployment in the Treatment of Carotid Blowout (Pampana, 2011)
Patient was admitted for massive pharyngeal hemorrhage, loss of consciousness and sphincter control. Angiography showed a severe narrowing of the left internal carotid artery and signs interpreted as bleeding from the left ECA branches. In addition, the angiography showed a reduced representation of left intracranial vessels without vascularization of the left ACA. Embolization of the small distal ECA branches was performed. Further angiography of the left external carotid artery was conducted highlighting media not coming from the distal ECA branches, so further left ICA angiography was performed. To control the hemorrhage and to restore a physiologic vessel patency with regular blood flow through the ICA, it was decided to deploy a covered stent graft. Originally a 6x20 mm Fluency stent was chosen, however it determined a high attrius with the vessel wall and to avoid an extension of the vessel laceration, it was decided to opt for a different stent with a lower profile and higher flexibility. A 6x15 mm Direct Stent was chosen. The stent was deployed at 6 atm successfully. An angiographic check revealed recovery of the vessel patency and blood flow, and the exclusion of the lacerated segment of the ICA with hemorrhage control, though a slight early venous filling and contrast material outlining the internal carotid artery along the skull base, due to iatrogenic dissection, persisted. Subsequent cerebral angiography showed an improvement in the perfusion of the MCA and its distal branches. As a clinical result, interruption of the rhinopharyngeal bleeding and normalization of the heart frequency and blood pressure was obtained, with resuscitation and stabilization of the patient’s condition.
Who We Are
Our partnerships are the foundation of our company. For the last 25 years our relationships with distributors and physicians have spanned over 40 countries on six continents. This global network makes it possible for patients to have more access to our live saving devices.
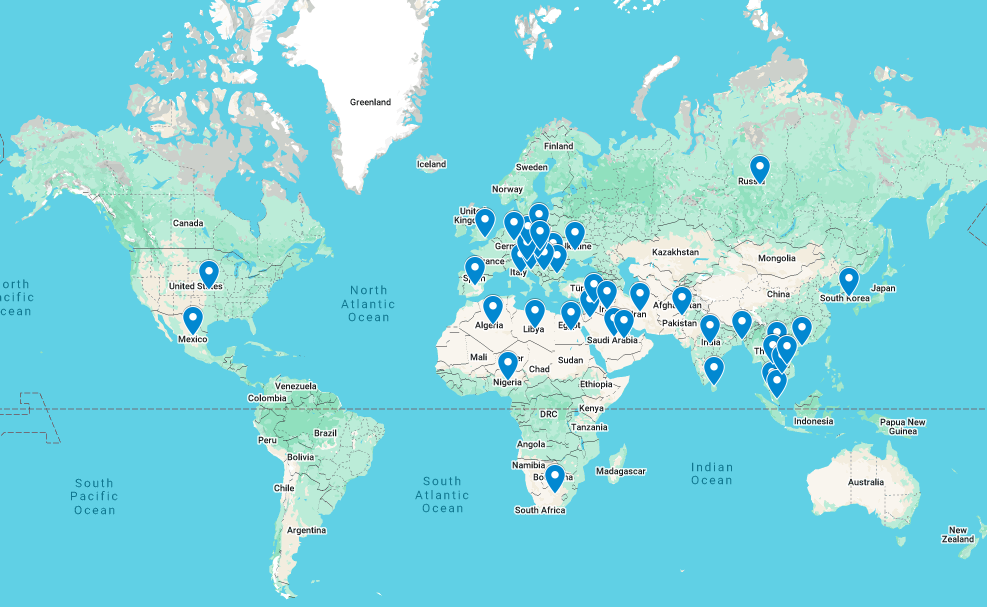
Implant Visuals of Our Devices
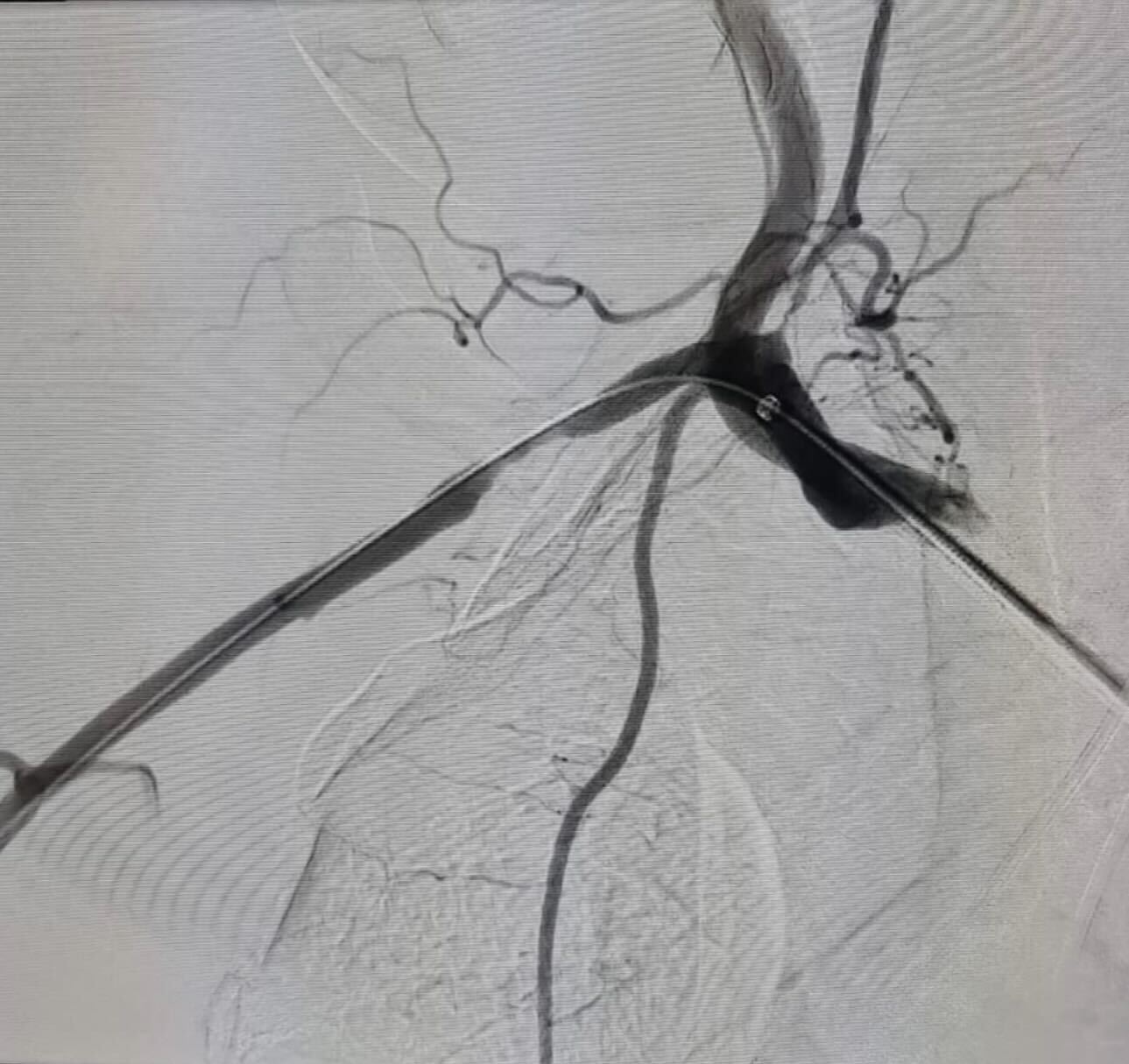
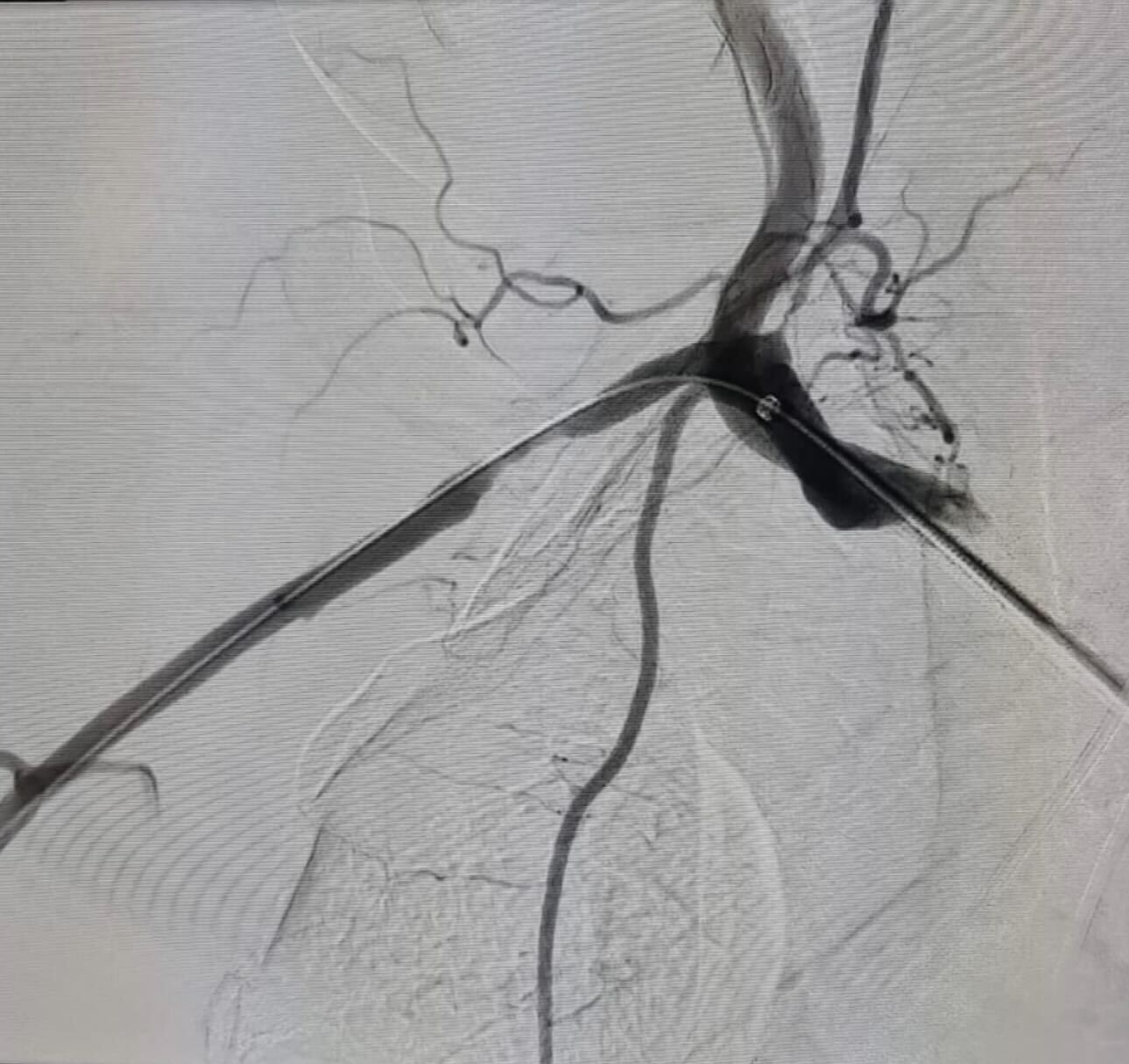
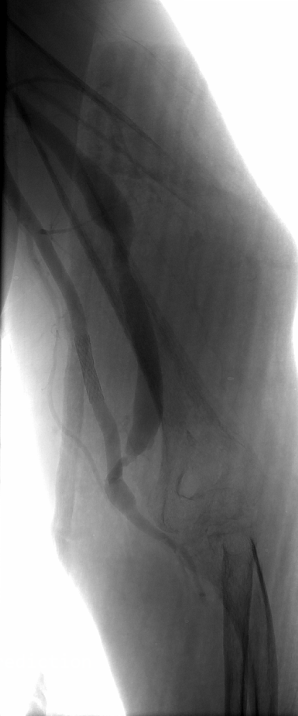
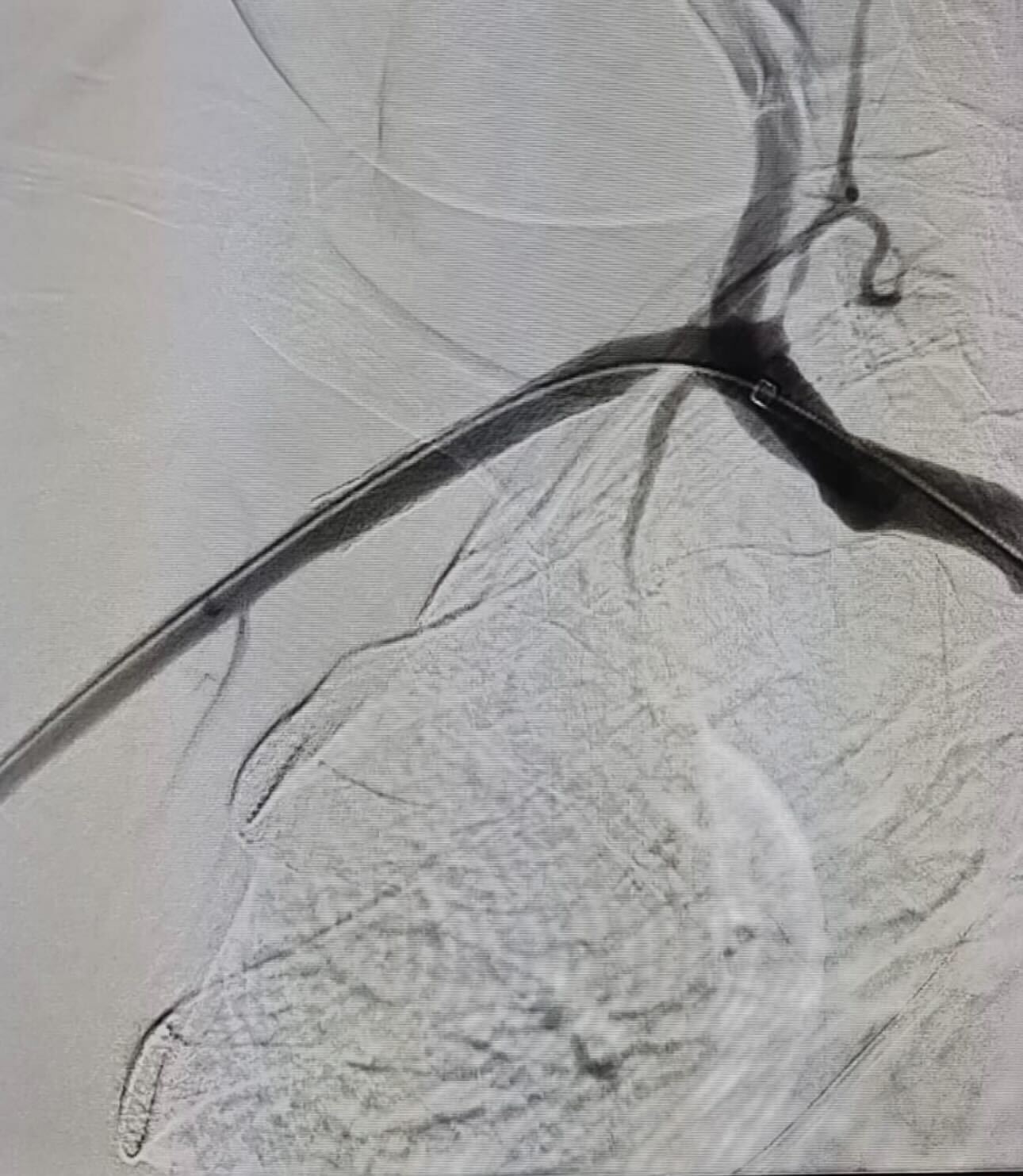


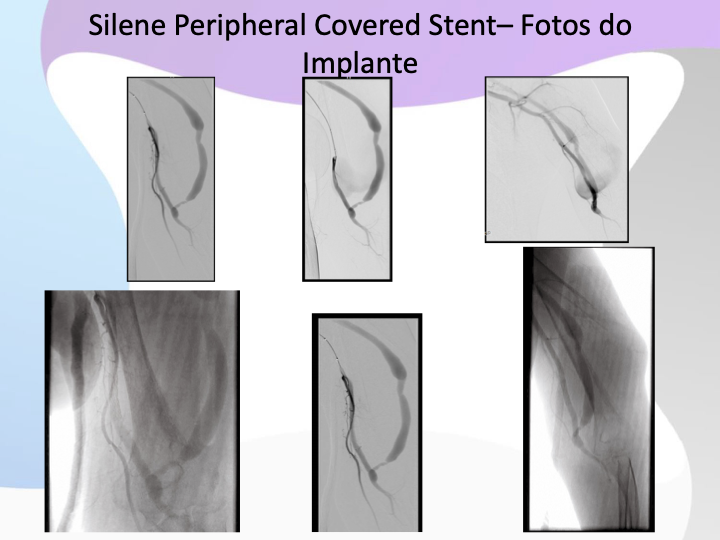
What We Do
InSitu Technologies® Inc. is a privately held medical device company specializing in intra-vascular stents and cardiovascular products. We are dedicated to providing the world with a full portfolio of state-of-the-art, minimally invasive products with a strong focus on the well being of our patients. Our engineers have been on the forefront of innovation and design with patented, proprietary designs for coronary and peripheral stenting. The unique design and ingenuity of our products is the direct result of collaboration with physicians from all corners of the world.


01
Design And Development
02
Manufacturing

03
Licensing
04
Contact us
Devices
View the full catalog of InSitu Technology’s innovative and precision manufactured products. We’ve spent the last 25 years building a solid portfolio of devices for cardiology and radiology. Our trusted devices have built a reputation on quality and design. We’ve also created a portfolio on essential devices to assist you in the cath lab.
25+
Year Experience
40+
Countries
219470+
Stents Implanted
190560+
PTCA balloon catheters
EuroPCR 2025
Paris, France
20-23 May 2025
A representative from InSitu Technologies will attend EuroPCR 2025 to explore future business opportunities and innovations in interventional medicine. This event will provide a platform for discussions on advancing cardiovascular solutions and strengthening industry collaborations.

hello@insitu-tech.com







Clinically Trusted
2025
Celosia™
Covered Stent
Silene™ Covered
Stent USA







